responder a perguntas sobre Do Dabs Stay In The System Longer e faça perguntas para obter ajuda de especialistas How Long Does a Dab Stay in Your System? – Marijuanalization BRAINSTACK
Login as registered user for prices, availability and discounts. United states pharmacopoeia (usp) solutions and reagents; O updated the gc method to include the new impurity: O system suitability solution is updated to include 200 µl/l of methanol and 1000.
Applications products services support. Eur. ) pure, pharma grade; Eur. ) pure, pharma grade; (c 3 h 8 o). Products are for research use or further manufacturing. Products are not for direct administration to humans or animals. 2. 5µl system suitability samples: System suitability solution and standard solution suitability requirements c resolution: Products are for research use or further manufacturing.
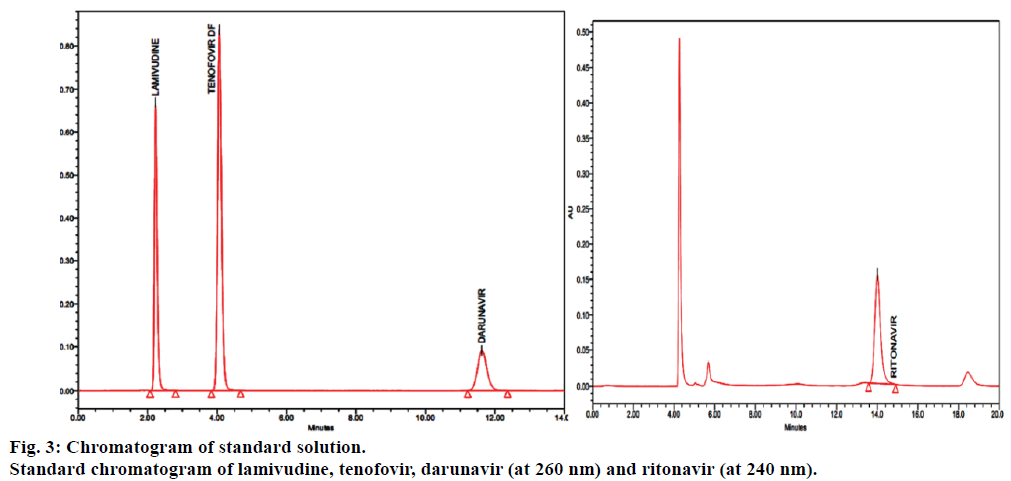
Simultaneous Quantification of Novel Antiretroviral Drug Combination by

WO1999024041A1 - Penetration enhancing and irritation reducing systems

WO1999024041A1 - Penetration enhancing and irritation reducing systems

WO1999024041A1 - Penetration enhancing and irritation reducing systems

Post a Comment
Post a Comment